
Robert Burns

The meat we sell in our shops is derived from skeletal muscle, the muscles that pull on bones of the skeleton to produce body movements. But, of course, there are a few of exceptions. The thin flat sheet of cutaneous muscle you see on a side of beef used to twitch the skin to keep flies off, and many major muscles do not pull directly on the skeleton, but achieve the same result by pulling on something else, like a large sheet of connective tissue. However, the characteristic and dominant feature of all this meat is its fibrous structure or texture.
This is a most important point for us in the meat trade. Meat texture is supremely important. Texturized vegetable protein, something that could be quite a commercial threat to us (simulated meat made from plant sources) has, so far, made little impact (apart from the chunks in some canned stews). This is because food technologists so far have been unable to extrude their plant proteins into anything resembling real meat. The taste and colour can be faked quite easily, but the texture cannot. In a way, therefore, it is the texture of meat, and the fact that many of our customers love to eat it, that keeps us all in business. Other topics with a practical importance are aging of meat and electrical stimulation of meat
In live animals, a sliding interaction of microscopic filaments enables a muscle to CONTRACT while, in meat, an ordered arrangement of
creates a characteristic texture that is difficult to imitate with processed plant proteins. These components of meat - fasciculi, fibres, fibrils and filaments - constitute a descending series with respect to size. Fasciculi are the largest units, while filaments are the smallest. The prefix "myo" may be used to create the terms myofibre, myofibril and myofilament, which are identical with muscle fibre, muscle fibril and muscle filament, respectively. If you hail from US, you ain't gonna like all this wierd English spelling of
The complex arrangement of muscle fasciculi is seen when meat is carved, and the same complexity is found in the muscle fibres that are bound into these bundles. Their complex arrangement is related to muscle function in the live animal. It used to be thought that muscles which contracted to a relatively small fraction of their resting length had fibres parallel to the long axis of the muscle, whereas muscles in which the strength of contraction was more important than the distance over which contraction occurred had fibres with an angular arrangement. Thus, it was thought that muscles might gain strength by leverage, but the contraction distance would be reduced. This was a nice idea, which many of us were taught, but really there is little or no evidence to support it, and a pennate (V-shaped fibre arrangement) structure actually may serve to enhance the overall range of muscle excursion.
If a small chunk of meat is placed under a dissecting microscope and teased apart with needles, the smallest fasciculi visible without magnification are composed of bundles of muscle fibres. Muscle fibres are the basic cellular units of living muscle and of meat. They are unusual cells because they are multinucleate (with many cell nuclei) and are extremely long (commonly several centimetres) relative to their microscopic diameter (usually less than one tenth of a millimetre).
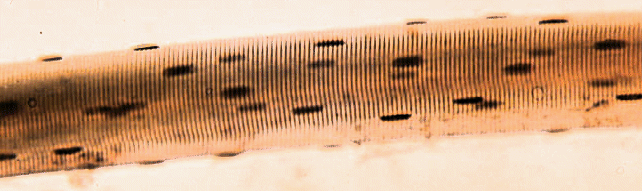
The image below shows a short part of one muscle fibre, but even this contains many nuclei (the dark blobs). The muscle fibres found in most commercial cuts of meat seldom
run the complete length of the muscle in which they are located. Individual fibres within a fasciculus may terminate at a point along the length of the fasciculus at a tapered ending anchored in the connective tissue on the surface of an adjacent fibre so that tapered endings transmit their force of contraction to the endomysium (the connective tissue around each muscle fibre) or directly to adjacent fibres through fibre to fibre junctions. The image below shows the endomysium in a transverse section of meat (the endomysium is black, the myofibrils are yellow).
Apart from tapered intrafascicular endings, the diameter of a fibre is assumed to be approximately constant along its length. Fibre diameters slowly increase during the growth of a muscle, but they also increase temporarily when a fibre contracts. Thus, when measuring fibre diameters in a growth study, special care must be taken to avoid or to correct for differences in the degree of muscle contraction.
The simplest way to examine individual muscle fibres is to place some meat fragments together with some water in a kitchen blender. After running the blender for a few seconds, the connective tissue holding the muscle fibres together is disrupted to leave a pale red suspension of broken muscle fibres in water. The red colour comes from myoglobin, a soluble red pigment found inside muscle fibres. The essential features of muscle fibre structure may be observed with an ordinary light microscope if a drop of the macerated muscle suspension is mounted on a microscope slide beneath a cover slip, as shown in the image below where, at the bottom of the frame, is an intact muscle fiber and above it is a smashed fiber with all its fibrils visible.
When meat animals are slaughtered, normally they are shackled and suspended from their hind limbs and some muscles, such as the filet or psoas muscles ventral to the vertebral column, become stretched. Other muscles, such as those in the posterior part of the hind-limb, are free from skeletal restraint and may contract weakly as the carcass becomes stiff after death (rigor mortis). If samples from stretched and contracted muscles are compared, transverse striations will appear relatively far apart in the stretched muscle and closer together in the contracted muscle.
If a drop of saturated sodium chloride solution is mixed with a drop of macerated muscle suspension, the muscle fibre fragments undergo some marked changes. Fibre fragments may slowly swell and disappear, or they may expand so violently that their interiors are extruded from their broken ends. Sarcomere length and the solubility of meat proteins in salt solutions are two commercially important properties of meat: meat with short sarcomeres tends to be tough, and salt-solubilized proteins are used to bind together the meat fragments in many
types of processed meat products.
Muscle fibres

 The transverse striations of fibres become visible if the iris diaphragm of the substage condenser is almost closed (the gain in contrast is offset by a loss of resolution, which is why the diaphragm is normally open wider). With a high magnification microscope objective, fibrils may be seen if they protrude from the broken end of a fibre or if they have escaped from a broken fibre. Under the surface membranes of muscle fibres may be seen some flattened bubble-like inclusions. These are the nuclei of the muscle fibre, and their DNA may be stained by treating the macerated muscle suspension with dyes such as haematoxylin. On the surfaces of any fibres that have retained some of their surrounding connective tissue may be seen branching capillaries, once part of the vascular bed of the muscle. Red blood cells (erythrocytes) may, or may not remain in the capillaries, depending on the efficiency with which the animal was stuck. If they do remain in the meat, they appear pale yellow in unstained preparations, and are often distorted or piled together like a stack of coins.
The transverse striations of fibres become visible if the iris diaphragm of the substage condenser is almost closed (the gain in contrast is offset by a loss of resolution, which is why the diaphragm is normally open wider). With a high magnification microscope objective, fibrils may be seen if they protrude from the broken end of a fibre or if they have escaped from a broken fibre. Under the surface membranes of muscle fibres may be seen some flattened bubble-like inclusions. These are the nuclei of the muscle fibre, and their DNA may be stained by treating the macerated muscle suspension with dyes such as haematoxylin. On the surfaces of any fibres that have retained some of their surrounding connective tissue may be seen branching capillaries, once part of the vascular bed of the muscle. Red blood cells (erythrocytes) may, or may not remain in the capillaries, depending on the efficiency with which the animal was stuck. If they do remain in the meat, they appear pale yellow in unstained preparations, and are often distorted or piled together like a stack of coins. The distance between the transverse striations is the sarcomere length.