
Gordon King, Animal Science, University of Guelph

Gordon King, Animal Science, University of Guelph
Biotechnology, although perceived by many as a very recent procedure, has been around almost throughout human history. Traditional applications used yeast to modify other materials as in the leavening of flour dough for making bread and the fermentation of barley to make beer or grapes to make wine. Other long established applications are the transformation of milk into cheese or yogurt, converting green plant material into silage and composting various materials for fertilizers. Quite recent discoveries in molecular biology such as the identification of restriction enzymes that cut DNA chains at specific sites (1970), methods for replicating genes (1973) and nucleotide sequencing (1977) provided tools for a "new" biotechnology involved with manipulating genes and moving these between species. Modern biotechnology perhaps originated with a 1980 decision by the US Supreme Court stating that " a live human-made microorganism is patentable matter." This ruling motivated commercial interest and research into searching for medical, agriculture and ecological applications.
The successful transfer of the growth hormone gene from rats to mice (Palmiter et al, 1982, Nature, Lond., 300:612-615 ) stimulated considerable interest and substantial research into development of new applications involving manipulation of subcellular components rather than complete organisms. To date, although an impressive body of new knowledge has been discovered and laboratory successes are numerous, biotechnology is still somewhat long on promises but short on performances that contribute to substantial improvements in commercial livestock farming. Nonetheless, conventional breeding is limited to reproductively compatible species and generation intervals. As the methodology for molecular genetics is refined, accelerated progress in genetic engineering should be possible once the proper genes are identified, appropriate markers are found, and expression of introduced genes can be regulated precisely in recipients. At present, much of the emphasis in livestock oriented programs focuses on acceleration of postnatal growth or enhancement of disease resistance with the expectation this would improve efficiency and profitability.
Another approach involves identifying and isolating the genes coding for specific proteins that are deficient in certain human diseases. These genes can then be inserted into livestock chromosomes. The ideal techniques allow regulating expression of the "foreign" gene or genes so they are only active in mammary tissue. Ewes, does or cows that successfully express the introduced trait would secrete the human protein in their milk, providing opportunity for isolation of the compound for therapeutic use. Similarly, antibodies or even genes from other species be introduced into chickens. The antibodies or pharmaceutical proteins resulting from such treatments are concentrated in the egg yolk and can be harvested for use in humans or other animals. Successes in any of these areas could create an entirely new range of animal products. However, any such pharmaceuticals must be produced under very stringent conditions and for a somewhat limited market.
Another aspect of biotechnology involves introduction of foreign genes into bacteria or hybridoma cell lines so these altered organisms will produce new compounds for harvest. Recombinant-derived porcine and bovine somatotrophin are now produced in this way, as are a number of antibodies and gene probes used in disease treatment or diagnosis. The genome of micro-organisms can be manipulated to reduce pathogenicity (the ability to cause disease) while enhancing antigenicity (the ability to stimulate immunity), providing methodology to create more effective vaccines. Perhaps even more futuristic and exciting is the prospect for genetic engineering rumen micro-organisms with enhanced ability to degrade cellulose. Other strains could possibly be developed to break down highly ligninized materials in vitro to liberate cellulose or even convert the base material to starch. Cereal grain and sugar cane production generates several billion tones of crop residue every year which, if treated to improve digestibility, would alleviate much of the livestock malnutrition commonly encountered in lesser developed regions. Recombinant DNA technology might also be used to produce relatively inexpensive phytases which could be incorporated into animal diets to make phosphorus, which is usually bound tightly into the plant tissue, more readily available for digestion.
With the recent breakthrough in large-scale biosynthesis, resulting in
availability of large quantities at reasonable prices, potential uses for
somatotrophin (ST) are now receiving considerable interest in production
agriculture. This hormone, produced by the anterior pituitary of all
vertebrates, influences growth and metabolism. An important economic trait
resulting from exogenous ST administration is, that while treated animals grow
faster, the growth is more efficient and the carcass yields more lean meat.
Thus, the increase in size does not result from fat deposition but is a true
increase in muscle tissue. Some consumer activists are attempting to prevent
acceptance of these agents by suggesting that biologically active residues
might remain in products from treated animals. This is not a valid concern
since proteins like growth hormone are complex molecules that, if ingested by
another animal, would be broken down to constituent amino acids rather than
absorbed as an intact protein. Also, since each species has its unique
molecular arrangement of its protein hormone structure, hormones from one
species would not be biologically active even if injected into any other
species, except in those cases where the donor and recipient species are
closely related on the phylogenetic tree (i.e. bovine r-bST has slight activity
in ewes or does but none in sows).
| Growth Rate |
Feed Conversion |
Fat Content |
|
| Boars | + 13% |
+ 19% |
- 22% |
| Barrows | + 16% |
+ 32% |
- 33% |
| Gilts | + 22% |
+ 34% |
- 36% |
Campbell et al. Journal of Animal Science,
67:177-186 (1989)
The fact that somatotrophin (growth hormone) is galactopoietic has been known for at least five decades. This hormone regulates the partitioning of available nutrients in favor of mammary tissue in lactating females so that more milk can be synthesized. Recombinant-derived bovine somatotrophin (r-bST) can now be produced in substantial quantities, promoting interest in practical applications. Many research trials have demonstrated increased milk yield (ranging from 5 to 40%) and feed efficiency (up to 24%) in treated cows. Injections commence around 10 or 12 weeks after calving and continue for 3 to 6 months. This allows cows to reach maximum daily yield before treatment starts. Thus, the administered hormone maintains production at or near peak amounts for prolonged periods rather than stimulating even higher degrees of synthesis.
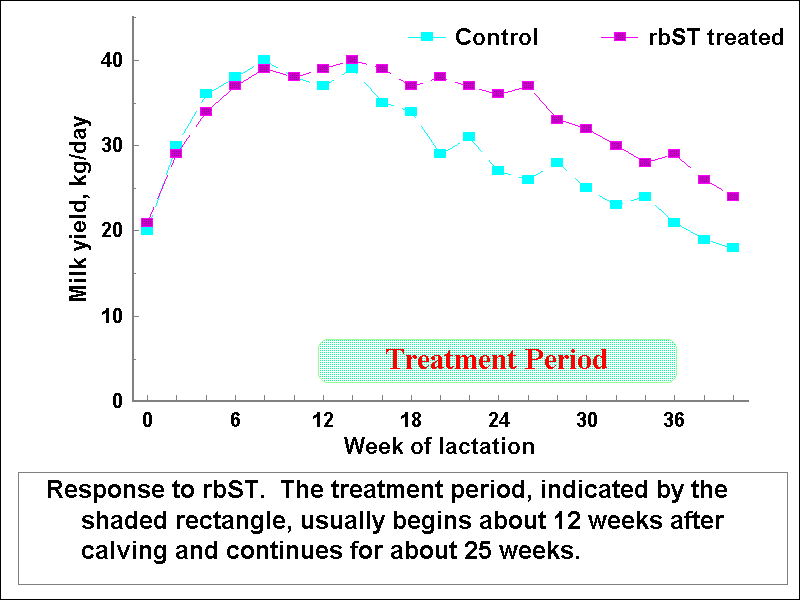
At present, recombinant bovine somatotrophin is approved for use in some
countries (e.g.. South Africa, Russia, USA, Mexico) but not in many others.
An even more imaginative programme is attempting to modify the histocompatability complex of pigs so their organs and tissues become suitable for transplantation into humans. Tissues removed from one part of the body and reattached to another site in the same individual (autografts) produce no imuno-rejection reaction. Similarly, grafts between identical twins or highly inbred animals are usually accepted because the donor and recipient have the same antigenic characteristics. In contrast, grafts between individuals of the same species (allografts) or different species (xenografts) will be rejected unless the natural immune responses are suppressed almost totally. Unfortunately, while this degree of suppression is possible, the currently available procedures for immunosuppression possess many undesirable side effects. To make the replacement process less hazardous and to generate a ready supply of organs for transplantation, a number of research laboratories seek techniques to produce donor animals, usually pigs, with modified antigenicity. One method under investigation involve insertion of human genes that alter the porcine major histocompatability complex so that it is similar to that of humans. Other approaches attempt to prevent recognition of or response to the specific foreign antigenic components contained in the transplant by a recipient's immune system. Although many problems must still be overcome before such procedures could become practical, it may eventually create a new and highly sophisticated livestock enterprise.
Safety
Practicality
Environment
Perhaps a major applications in the future will be modification of product composition and quality to suit consumer preferences (e.g. meat with reduced cholesterol or better keeping qualities). Scientists require considerably more knowledge about the biochemical pathways controlling cellular activities to fully exploit this aspect in animal commodities.
The Biotechnology Information Center at the National Agricultural Library of the US Department of Agriculture provides access to a variety of information services and publications relating to agricultural biotechnology.
The Canadian Food Inspection Agency Office of Biotechnology offers general information on food related biotechnology including the regulating of agricultural products, specific requirements for safety and for the environmental assessment plants, animal feeds and biofertilizers.
The Canadian Federation of Agriculture provides national and international information on biotechnology.
Environment Canada offers information on biotechnology as it relates to the Canadian Environmental Protection Act.
Health Canada's Office of Food Biotechnology is responsible for the regulatory process and safety assessment of genetically modified foods.
Information Systems for Biotechnology provides the results of numerous risk assessment experiments relating to agricultural biotechnology.